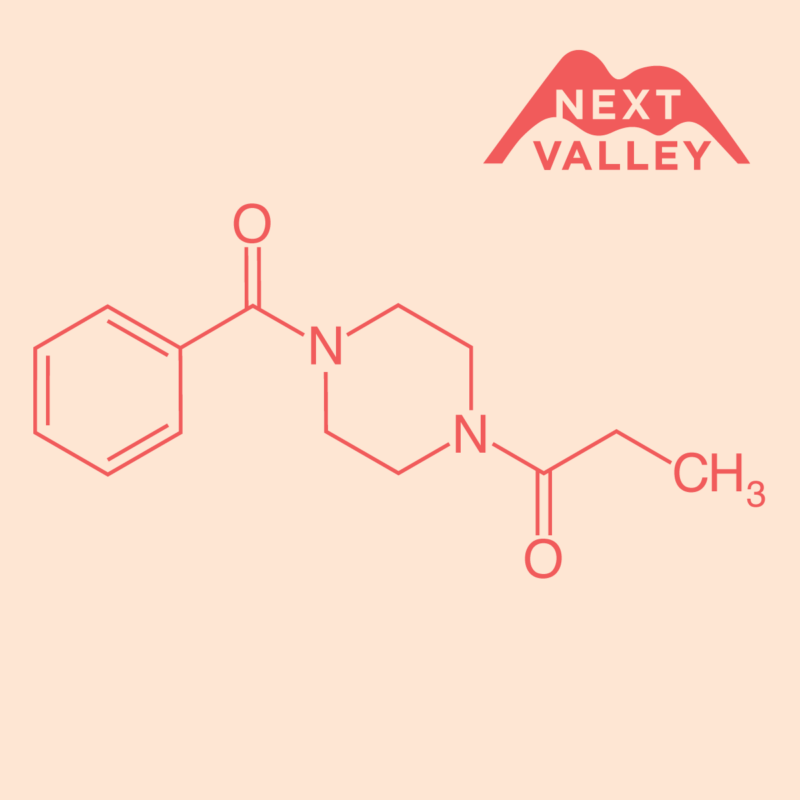
What is Sunifiram?
Sunifiram (also known as DM‑235) is a piperazine derivative that emerged from investigations into racetam‑like compounds. It was studied in the early 2000s for its structural relationship to other nootropics, focusing on potential mechanisms involving neurotransmitter modulation.
Use
We emphasize that this product is not permitted for consumption as food or as a food additive,
according to the guidelines of both the FDA and the European Commission! Advising on its
consumption, or selling or producing it for consumption, is strictly prohibited.
Keep out of reach of children!
Store securely closed in a cool, dry place. Keep away from direct sunlight and heat.
Next Valley aims to be a reliable provider of quality Nootropics. Certain Nootropics are not considered dietary supplements, and we want to provide our customers with clear information about this.
Content
Content per 1 HPMC capsule:
10 mg Sunifiram
Gluten-free organic rice flour (filler)
Magnesium stearate (anti-caking agent)
Hydroxypropyl methylcellulose (HPMC) capsule
Manufacture date: 20240527
Retest date: 20260526
Packing date: 20250326
Lot no.: 250326
10 gram Sunifiram Bulk powder
Manufacture date: 20240527
Retest date: 20260526
Packing date: 20250326
Lot no.: 250326
Bulk powder is supplied without a measuring scoop.
Certificate of Analysis
Potentially incorrect analysis certificates (COA’s) in the market for Non-Consumptive Substances! Read here -›
A Certificate of Analysis may sometimes be referred to as a COA, a CofA, a Certificate of Conformity, or a Certificate of Conformity. It is a document certifying that a delivered product meets the recipient’s specifications.
Certificate of Analysis for Sunifiram
The content of a Certificate of Analysis varies by industry and product category. Below, we discuss the general content of a COA as it applies to the raw materials used in Next Valley products.