What is Choline Bitartrate?
Choline Bitartrate is a compound of choline and tartaric acid, known for its role in supporting various bodily functions. Choline is an essential nutrient required by the body to produce cells, hormones, and neurotransmitters like acetylcholine. This supplement offers a concentrated source of choline and is especially popular among those looking to support cognitive function and liver health. While choline is an important nutrient, this does not automatically mean that all its claimed health benefits are recognized by the EU.
Discover the benefits of Choline Bitartrate from AvantVital
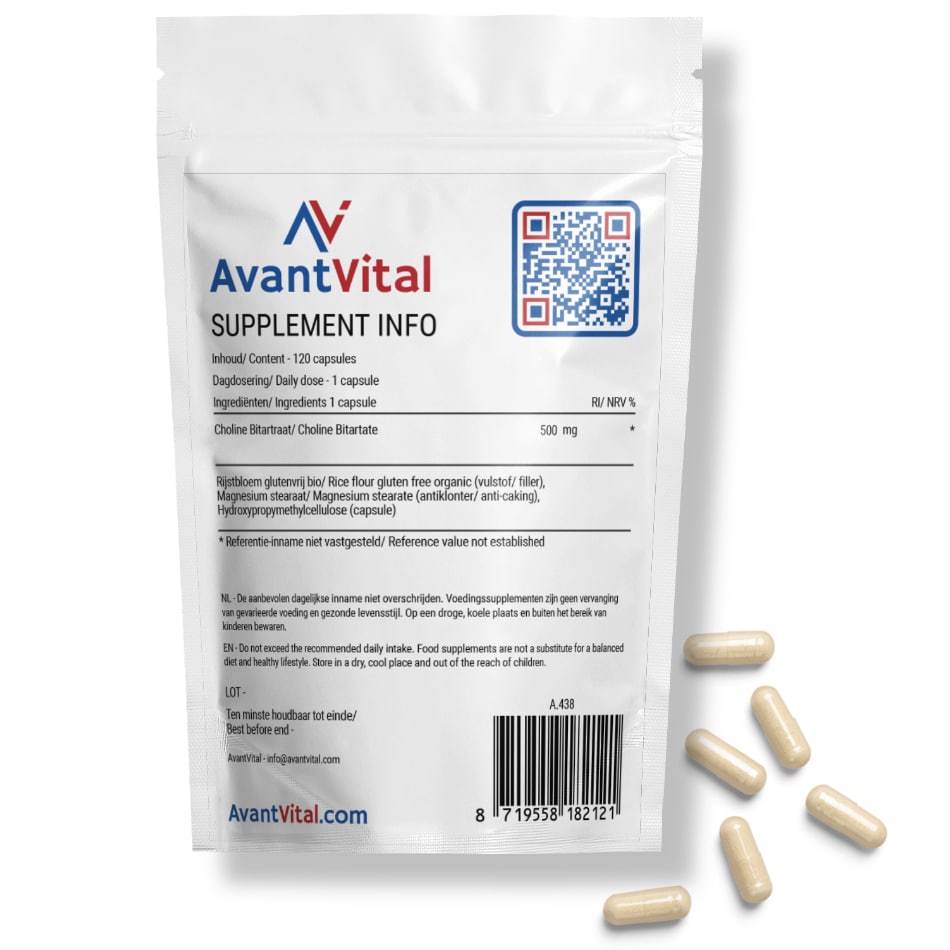
Choline Bitartrate from AvantVital provides 500 mg of choline per capsule. This supplement can be particularly beneficial for vegetarians, vegans, and others who may struggle to get sufficient choline from their diet.
Available in a convenient resealable pouch containing 120 capsules, each with 500 mg of Choline Bitartrate.
Features
Choline is an essential nutrient crucial for the production of phospholipids in cell membranes and the synthesis of acetylcholine, a neurotransmitter involved in cognitive function and memory. While the body can produce choline in limited amounts, most people meet their choline needs through food sources like eggs, liver, and nuts. Choline Bitartrate offers a plant-based and easily absorbable source of choline, ideal for those seeking extra support.
Why Choose Choline Bitartrate from AvantVital?
Highly dosed and easily absorbable
AvantVital’s Choline Bitartrate is highly dosed at 500 mg per capsule, ensuring an effective supplement to your daily choline intake. Each package contains 120 capsules, enabling consistent and easy supplementation. This product is crafted with a focus on quality and bioavailability.
Suitable for vegetarians, easy to swallow & free from unhealthy additives
Choline Bitartrate from AvantVital is encapsulated in vegan HPMC capsules. These capsules are easy to swallow, free from unnecessary additives, and ideal for vegetarians seeking to enhance their choline intake without animal products.
Usage
The recommended dosage of AvantVital’s Choline Bitartrate is 1 capsule per day, unless otherwise advised by a healthcare professional. Each capsule contains 500 mg of choline, making one capsule sufficient to support your daily requirements.
Choline Bitartrate can be taken with or without food, depending on what works best for you. However, some users prefer taking it with food to avoid stomach discomfort. The timing of intake is flexible and can be adjusted to suit your daily routine.
For long-term use of Choline Bitartrate, it is recommended to take a break after several months to evaluate the supplement’s effects on your body. Consult a healthcare professional if you are pregnant, breastfeeding, taking medications, or have a medical condition.
Ingredients
Side Effects
Choline Bitartrate is generally well tolerated, but in some cases, mild side effects like stomach discomfort or a fishy odor in the breath may occur. If severe side effects occur, discontinue use and consult a healthcare professional.
Interactions
Choline Bitartrate may interact with certain medications, especially at high doses. It is important to consult a doctor before using Choline Bitartrate if you are taking other medications.
Disclaimer
This product is not intended to diagnose, treat, cure, or prevent any disease. This supplement should not be used as a substitute for a varied and balanced diet and a healthy lifestyle. Do not exceed the recommended daily dose. Keep out of reach of children. Consult your doctor or healthcare provider before using this supplement, especially if you are pregnant, breastfeeding, taking medications, or have a medical condition.
When sharing information about wellness and health, we must adhere to the boundaries set by European and Dutch regulations. These restrictions are in place to protect you and ensure that no unfounded health claims are made. It is important to note that while traditional or non-Western medical practices have their own place and respect, EU legislation requires health claims to be based on recognized scientific evidence. Within these frameworks, we strive to be as informative as possible without exceeding the limits of the law.