What is Acetyl-L-Carnitine?
Acetyl-L-Carnitine, often abbreviated as ALCAR, is a bioactive form of L-carnitine, a semi-essential amino acid naturally found in the body. This amino acid plays an important role in transporting fats to the mitochondria, where energy is produced. Acetyl-L-Carnitine is known for its good bioavailability and is often praised as a valuable supplement, especially for vegetarians and vegans, as it is mainly found in animal products such as meat and dairy. If you follow a lifestyle with little to no animal products, supplementing with Acetyl-L-Carnitine 500 mg can be especially valuable.
Discover the benefits of Acetyl-L-Carnitine from AvantVital
AvantVital’s Acetyl-L-Carnitine offers a high dosage of 500 mg per capsule and is designed for optimal absorption by the body. Thanks to the acetyl binding, which provides protection against oxidation and enables more efficient cellular uptake, ALCAR can easily pass through the cell membrane and be quickly made available for the body.
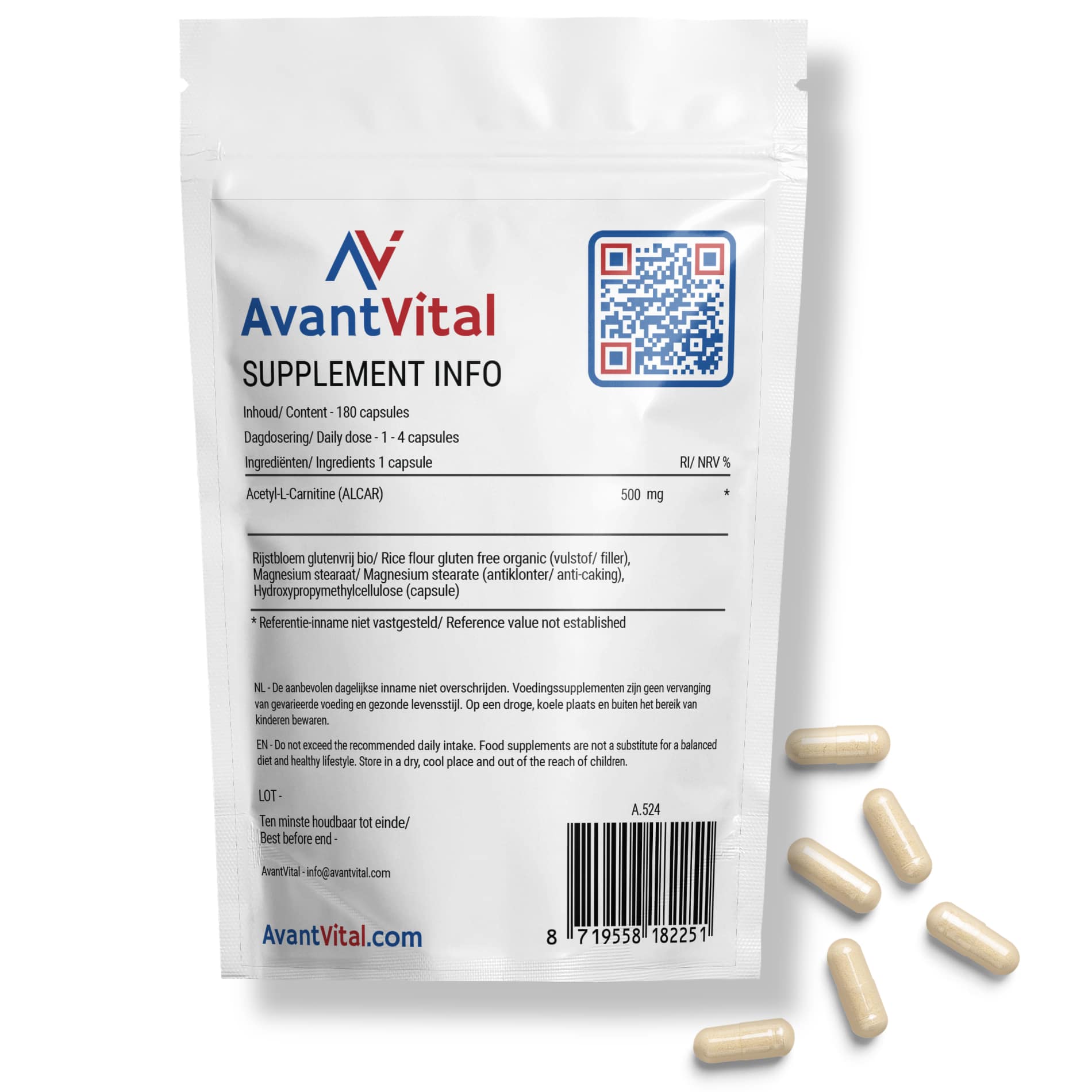
Available in a convenient resealable pouch with 180 capsules, each containing 500 mg of Acetyl-L-Carnitine.
Properties
Acetyl-L-Carnitine is the L-structured form of carnitine, which is recognized and well absorbed by the body. The addition of the acetyl group makes this form unique, as it supports rapid and efficient absorption in the body. This supplement contains only the L-form, the only natural and biologically available form of carnitine. As a result, Acetyl-L-Carnitine is easily processed and absorbed by the body, resulting in higher bioavailability and effectiveness compared to other forms of carnitine.
Why buy Acetyl-L-Carnitine from AvantVital?
High dosage and easily absorbable
Each capsule contains 500 mg of Acetyl-L-Carnitine, carefully formulated for a potent dose that can be tailored to your personal needs. AvantVital’s Acetyl-L-Carnitine stands out for its high quality and effectiveness, packaged in a resealable pouch with 180 capsules.
Acetyl-L-Carnitine vs. N-Acetyl-L-Carnitine
The terms “Acetyl-L-Carnitine” and “N-Acetyl-L-Carnitine” are sometimes used interchangeably but refer to the same compound. The “N-” prefix does not technically add anything to the function or structure of the product. The acetyl group is already bound to the nitrogen of the L-carnitine molecule, which is the most common and effective binding for use in supplements.
Usage
The recommended dosage of AvantVital’s Acetyl-L-Carnitine varies from 1 to 4 capsules per day, depending on individual needs and the advice of a health professional. Since each capsule contains 500 mg of ALCAR, you can adjust the dosage according to your preference.
Acetyl-L-Carnitine can be taken with or without food, although some users prefer to take it with food to avoid stomach discomfort. The timing of intake is flexible and can be adjusted to fit your daily routine, such as in the morning or during lunch.
Continuous use of Acetyl-L-Carnitine is possible, but for optimal results, it may be beneficial to take a break after a few months. This helps maintain effectiveness and provides a moment to evaluate how the supplement works for you.
Consult a health professional before starting Acetyl-L-Carnitine, especially if you are pregnant, breastfeeding, taking medications, or have a medical condition.
Ingredients
Side effects
Acetyl-L-Carnitine is generally well tolerated, but in some cases, mild side effects such as stomach discomfort or slight nervousness may occur. If severe side effects occur, discontinue use and consult a health professional.
Medication
Acetyl-L-Carnitine may interact with certain medications, such as thyroid medications and blood thinners. It is important to consult your doctor before using Acetyl-L-Carnitine if you are taking other medications.
Disclaimer
This product is not intended to diagnose, treat, cure, or prevent any diseases. This supplement should not be used as a substitute for a varied and balanced diet and a healthy lifestyle. Do not exceed the recommended daily dosage. Keep out of reach of children. Consult your doctor or healthcare provider before using this supplement, especially if you are pregnant, breastfeeding, taking medication, or have a medical condition.
When sharing information about well-being and health, we encounter limits set by European and Dutch regulations. These restrictions are there to protect you and ensure that we do not make unfounded health claims. It is important to note that, although traditional or non-Western medical practices have their own place and respect, EU legislation requires that health claims must be based on recognized scientific evidence. Within these frameworks, we strive to be as informative as possible, without crossing the boundaries of the law.